Abstract
Introduction: Immunotherapy is a promising therapeutic intervention for cancer treatment. Activation of the immune system via checkpoint blockade has been shown to produce antitumor responses in patients with both solid and hematological tumors. However, many patients do not respond to checkpoint inhibitors, and additional therapies are needed to treat these patients. Testing immunotherapies requires a functional human immune system; thus, it is difficult to evaluate their effectiveness using conventional experimental models. For this reason, establishing in vivo models that closely reproduce not only human tumors, but also their interactions with the human immune system, has become mandatory.
Methods: We developed a humanized mouse model and combined it with a patient-derived tumor xenograft (PDTX). Humanized mice (HuMice) were generated by transplantation of cord blood or mobilized peripheral blood CD34+ hematopoietic stem and progenitor cells into preconditioned immunodeficient mice. We compared human engraftment in 3 different mouse strains: NSG (NOD.Cg-Prkdc scidIl2rg tm1Wjl/SzJ), NSGS (NOD.Cg-Prkdc scidIl2rg tm1Wjl Tg(CMV-IL3,CSF2,KITLG)1Eav/MloySzJ) and NBSGW (NOD.Cg-Kit W-41J Tyr + Prkdc scid Il2rg tm1Wjl/ThomJ). Immune cell profiling and distribution was performed using flow cytometry and immunohistochemistry. The B cell receptor (BCR) repertoire was evaluated using an RNA-based NGS assay. To evaluate the maturation and functionality of T cells developing in HuMice we performed proliferation, degranulation and intracellular cytokine staining.
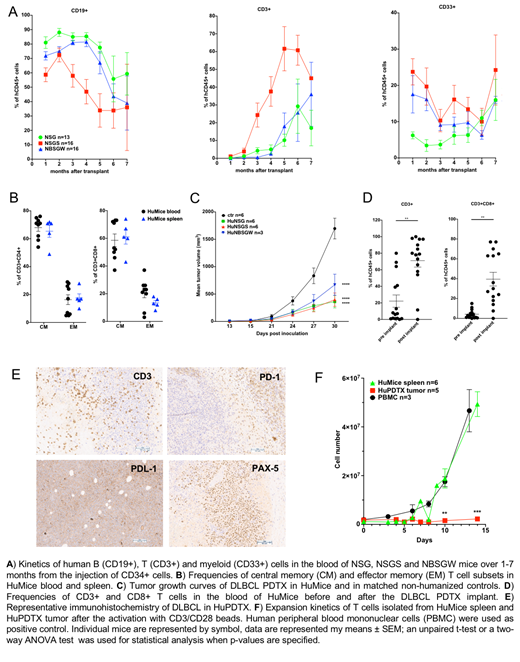
Results: Two months after CD34+ cell transplantation, we observed high levels of human hematopoietic chimerism in all the 3 strains. NSGS mice supported high-level chimerism as early as 1 month after transplantation, with more than 25% of human CD45+ cells in the blood. In all mice the majority of human circulating leukocytes were CD19+ B cells. An early appearance of CD3+ human T cells was detected in NSGS mice as compared to the other strains. Notably, the T cell expansion correlated with a decrease in relative B cell abundance while the myeloid cell contribution to the graft remained steady. We documented the differentiation of CD4+ and CD8+ human T cells at a 2:1 ratio. The characterization of the T cell subsets revealed that the majority was represented by CD45RA-CCR7- effector memory cells in both the spleen and the blood of HuMice. Nevertheless, recipient mice did not exhibit overt signs of graft-versus-host disease. We also evaluated the cytotoxic potential of T cells isolated from the spleen of HuMice: ex vivo peptide antigen (i.e. EBV) presentation let to generation of effective and specific cytotoxic T-cells.
After assessing a functional human immune system reconstitution in HuMice, we challenged them in vivo with low-passage tumor fragments from a diffuse large B cell lymphoma (DLBCL) PDTX. All tumor implants were successfully engrafted in both HuMice and non-humanized controls. Remarkably, all the 3 HuMice strains showed a significant reduction in the tumor volume and/or eradication compared to matched non-humanized controls. Flow cytometry analysis of the peripheral blood of humanized PDTX revealed that the tumor engraftment elicited a significant expansion of CD3+ T cells and cytotoxic CD8+ lymphocytes. Moreover, tumors developing in HuMice exhibited intermediate to high levels of tumor infiltrating T lymphocytes commingling with the neoplastic B cells, as determined by immunohistochemistry. Large areas of necrosis were often observed in PDTX of HuMice. Infiltrating CD3+ cells were TIGIT, PD-1 and Lag-3 positive, and did not efficiently proliferate ex vivo: all features consistent with an exhaustion phenotype. PDTX of HuMice often displayed larger areas of necrosis.
Conclusions: Collectively, our data demonstrate that a robust reconstitution can be achieved in different strains of immunocompromised mice and that HuMice elicit effective anti-lymphoma responses. PDTX HuMice represent a powerful platform to study host-tumor interactions, and to test novel immune-based strategies (CAR-T, bifunctional Abs) and new pharmacological approaches to counteract T-cell exhaustion.
Scandura: Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Constellation: Research Funding; MPN-RF (Foundation): Research Funding; CR&T (Foudation): Research Funding; European Leukemia net: Honoraria, Other: travel fees . Roth: Janssen: Consultancy; Merck: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal